New Windsor, NY 12553

Corneal Cross-Linking
Keratoconus is an eye condition in which the cornea weakens and thins over time, causing the development of a cone-like bulge and optical irregularity of the cornea. A rare condition, keratoconus typically first appears in individuals who are in their late teens or early twenties. It can result in significant visual loss and may lead to corneal transplant in severe cases.
In April 2016, the FDA approved Photrexa® Viscous (riboflavin 5'-phosphate sodium in 20% dextran ophthalmic solution) and Photrexa® (riboflavin 5'-phosphate sodium ophthalmic solution) and the Avedro KXL® System for corneal cross-linking, a minimally invasive outpatient procedure that combines the use of Vitamin B12 eye drops and ultra-violet (UV) light.
They are the first and only therapeutic products for corneal cross-linking which have been FDA approved to treat progressive keratoconus, and we offer them to patients in our practice. The approval of Photrexa Viscous, Photrexa and the Avedro KXL® System offers an effective treatment for patients who, until recently, had no therapeutic options to limit the progression of this sight-threatening disease.
What is Corneal Cross-Linking?
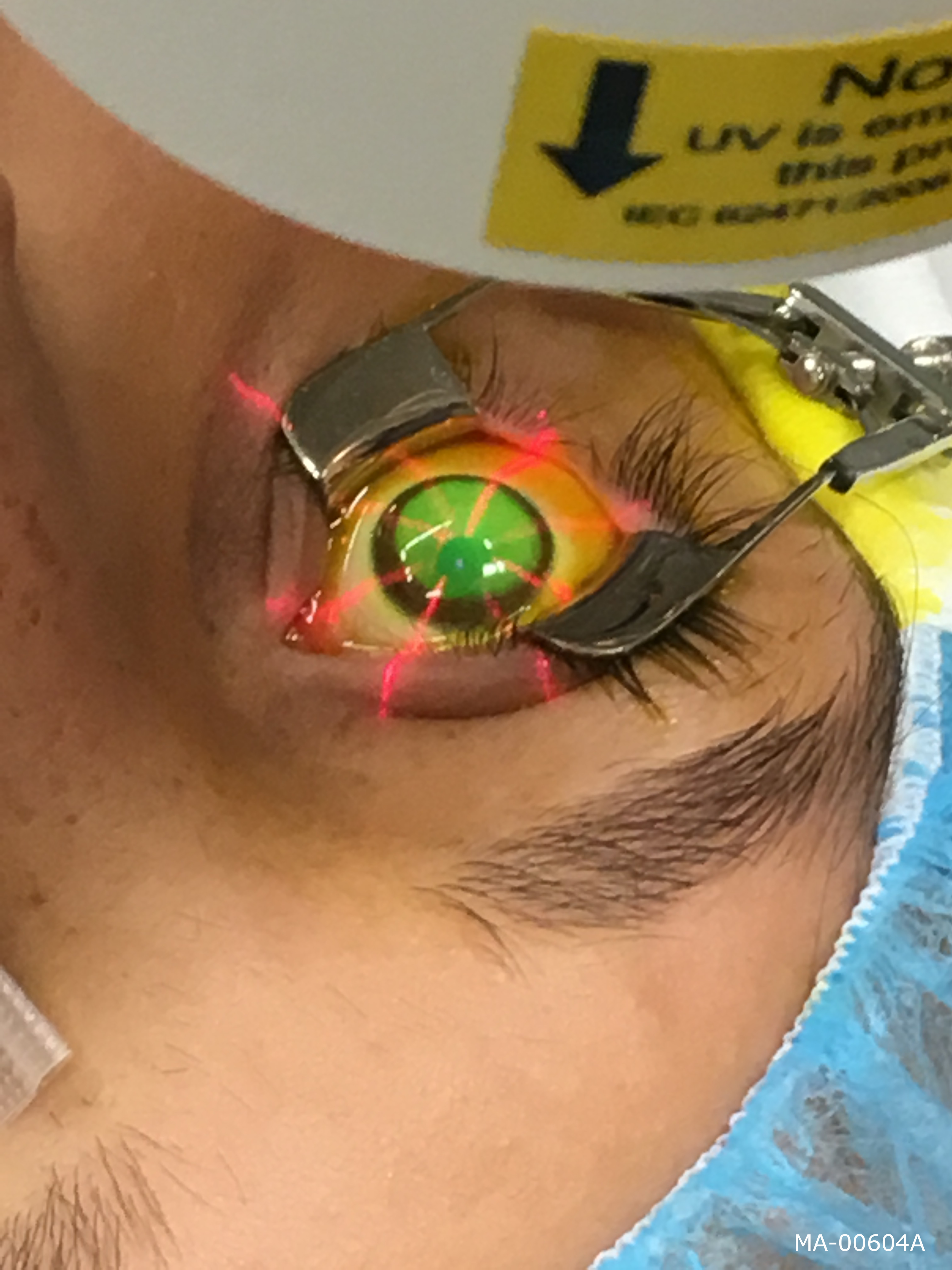
Corneal cross-linking is a minimally invasive outpatient procedure that combines the use of Photrexa® Viscous (riboflavin 5'-phosphate in 20% dextran ophthalmic solution), Photrexa® (riboflavin 5'-phosphate ophthalmic solution) and the Avedro KXL® System for the treatment of progressive keratoconus.
The safety and effectiveness of corneal cross-linking has not been established in pregnant women, women who are breastfeeding, patients who are less than 14 years of age and patients 65 years of age or older.
What are the side effects of corneal cross-linking?
- The most common ocular adverse reactions in any corneal cross-linked eye were haze (corneal opacity), inflammation (punctate keratitis), fine white lines (corneal striae), disruption of surface cells (corneal epithelium defect), eye pain, reduced sharpness of vision (visual acuity) and blurred vision.
What warnings should I know about corneal cross-linking?
- Ulcerative keratitis, a potentially serious eye infection, can occur.
- Your doctor should monitor you for resolution of epithelial defects if they occur.
Corneal Cross-Linking with Photrexa Viscous, Photrexa and the Avedro KXL® System
What can I expect during the procedure?
- Photrexa Viscous eye drops will be applied to the cornea for at least 30 min; Depending on the thickness of your cornea, Photrexa drops may also be required.
- The cornea is then exposed to UV light for 30 minutes while additional Photrexa Viscous drops are applied.
- After numbing drops are applied, the epithelium (the thin layer on the surface of the cornea) is gently removed.
What can I expect after the procedure?
- You should not rub your eyes for the first five days after the procedure.
- You may notice a sensitivity to light and have a foreign body sensation. You may also experience discomfort in the treated eye and sunglasses may help with light sensitivity.
- If you experience severe pain in the eye or any sudden decrease in vision, you should contact your physician immediately.
- If your bandage contact lens from the day of treatment falls out or becomes dislodged, you should not replace it and contact your physician immediately.